CE Expiration Date:
CEU (Continuing Education Unit): Credit(s)
AGD Code:
Educational aims and objectives
This clinical article aims to discuss clinical aspects of a microsurgery with associated modified tissue guide regeneration.
Expected outcomes
Endodontic Practice US subscribers can answer the CE questions to earn 2 hours of CE from reading this article. Take the quiz by clicking here. Correctly answering the questions will demonstrate the reader can:
- Identify some causes and possible treatment for bone defects.
- Identify some commonly used materials for guided bone regeneration.
- Recognize CBCT as a suitable and important tool for diagnosis, treatment planning, pre- and postoperative evaluation, and management of complex periradicular surgery.
- Identify some aspects of surgical treatment of a radicular cyst.
- Realize that persistent infection can occur after primary or even secondary root canal treatment, necessitating an apical surgery.
Drs. Maurício Paradella de Camargo, Tiago Braga, Murilo Priori Alcalde, Marco Antonio H Duarte, Rafael de Camargo, and Rodrigo Ricci Vivan look back on a microsurgery with associated modified tissue guide regeneration techniques after 5 years
Introduction
The aim of endodontic treatment is to treat apical periodontitis caused by infection of the root canal system.1 However, persistent infection can occur after primary or even secondary root canal treatment and could demand apical surgery to remove the infected site, favoring the apical healing.2
Bone defects are mainly caused by trauma, infections, congenital malformations, and post-surgical procedures.3,4 Although the human body has significant repair capacity, in cases of extensive bone lesions, the bone deposition cannot occur completely, forming a scar of fibrosis.2,3 This inadequate tissue growth is caused by the invagination of connective and/or epithelium tissue into the bone-defect area.2,4,5 Guided tissue regeneration (GTR) — using barrier membranes, bone grafts, and/or biomaterials on the bone defect created by infection and/or surgical techniques — has been widely used to avoid the epithelium invagination area and improve the healing of the surgical area.6,7 Currently, the most common materials used for GTR in the surgical procedures are bone replacement grafts, barrier membranes, and host modulating agents such as platelet-rich plasma (GRP).8-11
Platelet-rich plasma is the result of laboratory processing of autologous blood collected in the preoperative period. It consists of a group of polypeptides in which the growth factors released by platelets form a group of biological mediators that regulate important cellular events in tissue repair and/or cell proliferation. These include differentiation, chemo taxis, and matrix formation.10-12
CBCT is a suitable and important tool for diagnosis, treatment planning, pre- and postoperative evaluation, and management of complex periradicular surgery.13,14 In addition, CBCT provides a three-dimensional analysis of a periradicular lesion and prevents damage of anatomical structures, which cannot be viewed on two-dimensional images of conventional radiography.13-15 The purpose of this clinical case report is to describe a microsurgery with associated modified tissue guide regeneraton techniques performed to treat an extensive periradicular lesion with a successful clinical, radiographic, and tomographic outcome after 5 years of follow-up.
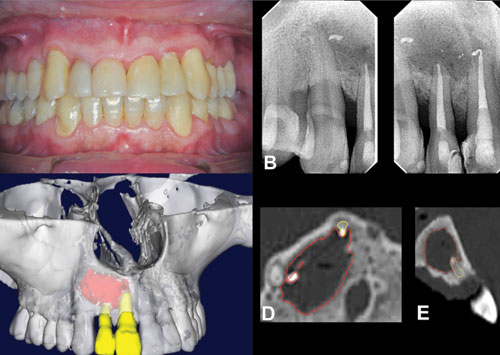
Figure 1: Periapical radiograph of the persistent periapical lesion after 1 year of root canal treatment 1A. 3D reconstruction of CBCT images. Note the size of the periapical lesion in axial. 1B. and 1C. Sagittal CBCT images. 1D. and 1E. The red lines show the extension of the lesion
Figure 2: The case after the enucleation of the periapical lesion, apicectomy, and retropreparation. 1A. Retrofilling material being performed on the teeth Nos. 7 and 8. 2B. Surgical cavity placement with HA nanoparticle + PLGA+ PRP. 2C. PPP barrier placed on the surgical cavity. 2D. Clinical view after suture procedure with Vycril® 6.0
Materials and methods
Case report
A 45-year-old male patient came to the dental office reporting a mild pain during palpation on the maxillary right lateral incisor (No. 7) and maxillary right central incisor (No. 8). During clinical exams, there was no swelling or any visual alteration in the region of the maxillary right lateral incisor (tooth No. 7) and maxillary right central incisor (tooth No. 8 ) (Figure 1A). After the clinical examination, the periapical radiographic exams detected the presence of a periapical lesion involving the previously reported teeth. The cold-thermal pulp test (Roeko Endo Frost, Langenau, Germany; Coltène/Whaledent Inc., Cuyahoga Falls, Ohio, USA) gave a nonvital result for both teeth, and periapical percussion and palpation tests indicated sensitivity.
Root canal treatment was performed with a crown-down technique using rotary system files, and irrigation was performed with 2.5% of sodium hypochlorite (NaOCl) through a 30 gauge needle (NaviTip®) (Ultradent, South Jordan, Utah) coupled to a 5 ml disposable syringe (NaviTip). After the root canal preparation, ultrasonic activation with 17% EDTA and 2.5% NaOCL solutions was performed 3 times for 20 seconds in each tooth.
Afterward, the root canals were dried, and an intracanal dressing with calcium hydroxide paste (Biodinâmica, Ibiporã, PR, Brazil) was used, and the teeth were coronally sealed with Coltosol® (Coltène/Whaledent Inc., Cuyahoga Falls, Ohio, USA) and composite. After 21 days, the intracanal dressing was removed by copious irrigation with saline solution, the root canals were dried, and the obturation was performed using gutta-percha points and AH Plus® (Dentsply Maillefer, Tulsa, Oklahoma, USA) with lateral compaction technique, and the coronal sealing was performed with composite resin.
After 12 months of follow-up, the symptoms disappeared; however, the periapical lesion was not healed (Figure 1B). Thus, the patient was then recommended for endodontic microsurgery. A CBCT (i-CAT™ [voxel dimension 0.2 mm]; Imaging Sciences International, Hatfield, Pennsylvania) was requested due to the dimension of the periapical lesion. The CBCT images showed an extensive periapical lesion involving the mesial portion of the maxillary central right incisor (tooth No. 8) until the mesial portion of the maxillary right canine (tooth No. 6) (Figures 1C and 1D). In addition, substantial bone loss was observed in the buccal-palatal direction without cortical bone loss. In the clinical examination, there was no buccal or palatal swelling.
The decision was made to proceed with endodontic surgery. Fifteen minutes before the surgery, the biochemist specialist collected 35 ml of the patient’s blood to process the PRP. For extraoral antisepsis, 2% chlorhexidine was used and local anesthesia with 3% mepivacaine with epinephrine 1:100,000 (Mepiadre DFL). The incision procedure was performed with a micro blade No. 67, and a full-thickness mucoperiosteal triangular flap was reflected at the base of the papillae, extending from the maxillary right central incisor to the left maxillary right canine. The vertical incision was placed on the distal side of the right maxillary canine.
There was no cortical bone loss; thus, the osteotomy was performed with a slow-speed spherical bur under copious saline solution irrigation. The periapical lesion was completely enucleated, and the granulation tissue was sent to histopathology. The apicectomy was performed on the maxillary right lateral incisor (tooth No. 7) and the maxillary right central incisor (tooth No. 8) with drill 699 under saline irrigation. A retrograde cavity preparation of the teeth was done with 5 mm of extension using a Berutti ultrasonic tip (EMS [Electro Medical System], Switzerland). The surgical cavity and the root canals were placed with methylene blue 0.005% (DMC Brazil; DMC USA, Plantation, Florida), and photodynamic therapy was applied. The methylene blue was removed with copious saline solution irrigation, and the canals were dried with sterile paper points and retrofilled with Sealapex™ sealer (Kerr Dental, Orange County, California) mixed with MTA (ProRoot® MTA, Dentsply Maillefer, Tulsa, Oklahoma, USA) (Figure 2A).
To prevent an undesirable fibrous repair, the PRP was mixed with hydroxyapatite nanoparticle + Lactic acid-co-glycolic acid (PLGA) (DMC Brazil; DMC USA, Plantation, Florida), and the surgical cavity was completely filled (Figure 2B). In order to protect the filling material, a membrane of platelet-poor plasma (PPP) was used on top of it (Figure 2C). The flap was repositioned after incising the periosteum at the base of the flap to obtain tension-free closure, and a suture was performed with VICRYL® 6.0 (Ethicon) (Figure 2D). For all procedures, a range of 3x to 25x magnification was used.
The histopathology results confirmed the presence of a radicular cyst. The clinical and radiographic assessment 5 years postoperatively confirmed a stable outcome (Figures 3A and 3B). CBCT showed the presence of the bone graft filling the surgical cavity (Figures 3B and 3C). The tissue appeared well integrated, and a similar radiopaque and granular is compared with the adjacent bone.
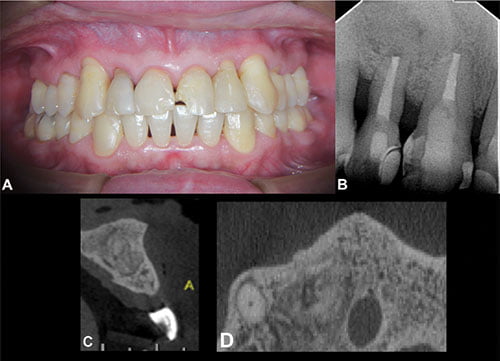
Figure 3: 3A. Clinical image of the patient after 5 years of follow-up. 3B. Periapical radiograph of the patient after 5 years of follow-up, showing absence of periapical lesion. 3C. and 3D. Axial and sagittal CBCT images. Both show the suitable healing of the periapical tissues
Results and discussion
Nonsurgical endodontic treatment should be the first choice of a persistent apical periodontitis.16 However, the treatment can fail because of intra- and/or extraradicular infections.1,2 The persistence of an infection concurs to the development of a radicular cyst.2,3,17 Although, in most cases, small cystic lesions repair after endodontic treatment, in the large lesions, an apical surgery may be needed.18,19 The histopathological examination confirmed the presence of radicular cyst, which explains the non-resolution after 1-year post-endodontic treatment and supports the surgical intervention.
The surgical treatment of a radicular cyst involves the debridement of granulation tissue, apical root resection, and retro filling of root apex.18-20 The retro filling was performed using the Sealapex mixed with MTA ProRoot. The Sealapex, a calcium hydroxide-based root canal sealer, can also be used as root-end filling material.
However, it is recommended to incorporate zinc oxide to provide better consistency and facilitate the clinical use.21,22 In this case, we used the Sealapex mixed with MTA to promote slight periapical inflammation, presence of fibrous capsule, and formation of new cementum in many cases.22,23 CBCT showed a satisfactory periapical healing, and the radiographic examination showed periodontal space formation.
The bone repair after apical surgery depends on the blood clot, angiogenesis of vessels, source of undifferentiated cells, space maintenance, and stability of the wound.24 Currently, GTR with the use of barrier membranes, bone grafts, and/or biomaterials to fill the bone defects has been widely used to improve the healing of surgical area.6-8
Synthetic calcium phosphate ceramics, HA, and tricalcium-phosphate are bio-compatible and have a composition and structure similar to the mineral part of bone tissue. These materials have been used to fill bone defects due to their osteo-conductive action.7-9 Regular ceramics have low tensile strength; thus, the association of HA dispersed on support substrate is indispensable.25
In this case, we combined PLGA with HA to fill the bone surgical defect. Previous studies reported that the association of the PLGA with HA increases mechanical properties, reduces degradation of the copolymers, helps maintain pH, increases the absorption of proteins, and improves the adhesion and growth of osteoblasts inside these hybrid frameworks.26,27
The PRP favors fibrin formation, improving the osteoconduction of the bone graft.11,28,29 Marx, et al.,20 showed that association of bone grafts with PRP increases 50% in the consolidation and mineralization and with an improvement of 15% to 30% in the density of the trabecular bone as a result of its osteoprogenitor properties. In this case, the surgical bone defect was filled with PRP + HA + PLGA to support the overlying membrane and also potentially as a scaffold to favor the osteoconductive properties for new bone formation.
Resorbable membranes have been used as a barrier to provide a wound stabilization after apical surgery, which can provide a wound stabilization during 6 to 8 weeks.9,31 In this case, the PPP gel was used as an alternative to conventional membranes. Previous studies showed that PPP is rich in fibronectin, which is known to increase cell proliferation and healing.32,33
Conclusion
The apical surgery in combination with GTR and PRP membrane led to a successful clinical, radiographic, and tomographic outcome after 5 years of follow-up, resulting in no gingival resection, the absence of bone defects, and hard tissue formation on the apical tissues.
References
- Siqueira JF Jr. Aetiology of root canal treatment failure: why well-treated teeth can fail. Int Endod J. 2001;34(10):1-10.
- Nair PN, Sjögren U, Figdor D, Sundqvist G. Persistent periapical radiolucencies of root-filled human teeth, failed endodontic treatments, and periapical scars. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87(5):617-627.
- Nair P. On the causes of persistent apical periodontitis: a review. Int Endod J. 2006;39(4):249-281.
- Lin LM, Rosenberg PA. Repair and regeneration in endodontics. Int Endod J. 2011;44(10):889-906.
- Ducheyne P, Mauck RL, Smith DH. Biomaterials in the repair of sports injuries. Nat Mater. 2012;11(8):652-654.
- Corbella S, Taschieri S, Elkabbany A, Del Fabbro M, von Arx T. Guided tissue regeneration using a barrier membrane in endodontic surgery. Swiss Dent J. 2016:126(1):13-25.
- Amini AR, Laurencin CT, Nukavarapu SP. Bone tissue engineering: recent advances and challenges. Crit Rev Biomed Eng. 2012;40(5):363-408.
- Taschieri S, Corbella S, Tsesis I, Bortolin M, Del Fabbro M. Effect of guided tissue regeneration on the outcome of surgical endodontic treatment of through-and-through lesions: a retrospective study at 4-year follow-up. Oral Maxillofac Surg. 2011;15(3):153-159.
- von Arx T, Al Saeed M. The use of regenerative techniques in apical surgery: a literature review. Saudi Dent J. 2011;23(3):113-127.
- Meschi N, Castro AB, Vandamme K, Quirynen M, Lambrechts P. The impact of autologous platelet concentrates on endodontic healing: a systematic review. Platelets. 2016;27(7):613-633.
- Qiu G, Shi Z, Xu HHK, et al. Bone regeneration in minipigs via calcium phosphate cement scaffold delivering autologous bone marrow mesenchymal stem cells and platelet-rich plasma. J Tissue Eng Regen Med. 2017; In press. doi: 10.1002/term.2416.
- Sánchez-Torres A, Sánchez-Garcés MÁ, Gay-Escoda C. Materials and prognostic factors of bone regeneration in periapical surgery: a systematic review. Med Oral Patol Oral Cir Bucal. 2014;19(4):e419-e425.
- Patel S, Durack C, Abella F, Shemesh H, Roig M, Lemberg K. Cone beam computed tomography in endodontics — a review. Int Endod J. 2015;48(1):3-15.
- Bornstein MM, Lauber R, Sendi P, von Arx T. Comparison of periapical radiography and limited cone-beam computed tomography in mandibular molars for analysis of anatomical landmarks before apical surgery. J Endod. 2011;37(2):151-157.
- Huumonen S, Ørstavik D. Radiological aspects of apical periodontitis. Endodontic Topics. 2002;1(1):3-25.
- Barone C, Dao TT, Basrani BB, Wang N, Friedman S. Treatment outcome in endodontics: the Toronto study — phases 3, 4, and 5: apical surgery. J Endod. 2010; 36(1):28-35.
- Sagit M, Guler S, Tasdemir A, Akf Somdas M. Large radicular cyst in the maxillary sinus. J Craniofac Surg. 2011;22(6):e64-e65.
- Martin SA. Conventional endodontic therapy of upper central incisor combined with cyst decompression: a case report. J Endod. 2007;33(6):753-757.
- Kourkouta S, Bailey GC. Periradicular regenerative surgery in a maxillary central incisor: 7-year results including cone-beam computed tomography. J Endod. 2014;40(7):1013-1019.
- Bashutski JD, Wang HL. Periodontal and endodontic regeneration. J Endod. 2009;35(3):321-328.
- Cunha SA, Rached FJ Jr, Alfredo E, León JE, Perez DE. Biocompatibility of sealers used in apical surgery: a histological study in rat subcutaneous tissue. Braz Dent J. 2011;22(4):299-305.
- Tanomaru-Filho M, Luis MR, Leonardo MR, Tanomaru JM, Silva LA. Evaluation of periapical repair following retrograde filling with different root-end filling materials in dog teeth with periapical lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102(1):127-132.
- Bernabé PF, Holland R, Morandi, et al. Comparative study of MTA and other materials in retrofilling of pulpless dogs’ teeth. Braz Dent J. 2005;16(2):149-155.
- Torabinejad M, Watson TF, Pitt Ford TR. Sealing ability of a mineral trioxide aggregate when used as a root end filling material. J Endod. 1993;19(12):591-595.
- Boyapati L, Wang HL. The role of stress in periodontal disease and wound healing. Periodontol 2000. 2007:44:195-210.
- Sui G, Yang X, Mei F, et al. Poly-L-lactic acid/hydroxyapatite hybrid membrane for bone tissue regeneration. J Biomed Mater Res A. 2007;82(2):445-454.
- Tsuruga E, Takita H, Itoh H, Wakisaka Y, Kuboki Y. Pore size of porous hydroxyapatite as the cell-substratum controls BMP-induced osteogenesis. J Biochem. 1997;121(2):317-324.
- Motta AC, Duek EAR. Synthesis, characterization, and “in vitro” degradation of poly (L-lactic acid-co-glycolic acid), PLGA. Matéria (Rio J). 2006;11(3);340-350.
- Lozada JL, Caplanis N, Proussaefs P, Willardsen J, Kammeyer G. Platelet-rich plasma application in sinus graft surgery: Part I — Background and processing techniques. J Oral Implantol. 2001;27(1):38-42.
- Liu Y, Kalén A, Risto O, Wahlström O. Fibroblast proliferation due to exposure to a platelet concentrate in vitro is pH dependent. Wound Repair Regen. 2002;10(5):336-340.
- Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62(4):489-496.
- Douthitt C, Gutmann JL, Witherspoon DE. Histologic assessment of healing after the use of a bioresorbable membrane in the management of buccal bone loss concomitant with periradicular surgery. J Endod. 2001;27(6):404-410.
- Simonpieri A, Del Corso M, Vervelle A, et al. Current knowledge and perspectives for the use of platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) in oral and maxillofacial surgery part 2: Bone graft, implant and reconstructive surgery. Curr Pharm Biotechnol. 2012;13(7):1231-1256.
- Gubina B, Rožman P, Bišcević M, Domanović D, Smrke D. The influence of allogeneic platelet gel on the morphology of human long bones. Coll Antropol. 2008;38(3):865-870.
Stay Relevant With Endodontic Practice US
Join our email list for CE courses and webinars, articles and more..


