CE Expiration Date:
CEU (Continuing Education Unit): Credit(s)
AGD Code:
Educational aims and objectives
This clinical article aims to explain the advantages of the combined use of mechanical instrumentation, ultrasonics, and magnification in root canal treatments and retreatments.
Expected outcomes
Endodontic Practice US subscribers can answer the CE questions to earn 2 hours of CE from reading this article. Take the quiz by clicking here. Correctly answering the questions will demonstrate the reader can:
- Identify the main reason of failures in root canal treatments.
- Recognize the main applications of ultrasonics in endodontics.
- Recognize the importance of preparing and cleaning isthmuses, flattened areas, and secondary root canals.
- Identify the clinical protocols to perform a thorough cleaning of the whole root canal system.
- Realize the obturation removal in retreatments without the use of solvents and complying with a minimally invasive approach.
Dr. Alexandre Capelli with co-authors Drs. M.A.H. Duarte, R. Vivan, M. Camargo, and F. Quintela discuss the advantages of the combined use of mechanical instrumentation, ultrasonics, and magnification in root canal treatments and retreatments
Conventional endodontics advocates that treatments should be done performing cleaning and shaping procedural steps in the root canal. Currently, with the widespread use of nickel-titanium instruments, the shaping process has become faster and safer; however, studies have shown that up to 35% of the canal walls remain untouched during the biomechanical shaping stage.1-3 The use of ultrasonic tips improves efficiency of treatment and should be considered as an adjunct to how treatment is rendered to improve quality of care, especially on surfaces and regions where conventional instruments do not reach, considering that many teeth have isthmuses and flattened areas.1-4
The use of ultrasonic tips under optical magnification (either using glasses or microscope) and proper lighting is a safe and effective way to address a great number of endodontic clinical situations.
It can be applied to the following:
- Removing calcified nodules inside the pulp chamber
- Refining access and searching for cal-cified and/or hidden canals (Figure 1)
- Removing separated instruments and other obstructions inside the root canal
- Removing intraradicular posts
- Preparing isthmus and flattened areas
- Agitating root canal irrigants and disinfectants
- Activating calcium hydroxide and root canal sealer
- Root canal obturation removal within the canal system
- Cutting and condensing gutta percha
- During periapical surgery, for osteotomy, apicoectomy, and retropreparation procedures
The piezoelectric ultrasonic unit is a remarkable tool, indispensable for its precision and effectiveness, with potential to be incorporated as the main tool in most steps of the root canal treatment and
retreatment.

Figure 1: Locating calcified canals using a pear-shaped diamond-coated ultrasonic tip (E6D diamond-coated ultrasonic tip, Helse Ultrasonic, Santa Rosa de Viterbo, Brazil; GEFD-1 ultrasonic tip, Vista Dental, Racine, Wisconsin; or Pear Diamond ultrasonic tip, Excellence in Endodontics, San Diego, California.)
The ultrasonic tip
The ultrasonic tip is a metal insert that will actuate directly on the terminal end of the tip where it contacts tooth structure. A tip is selected for a specific application, according to the geometry and utilization of the tip. An ultrasonic tip can be of many different shapes and angles, and made for different piezo units, but one main characteristic that must be considered is whether or not it is diamond coated.
Diamond-coated tips
Diamond-coated tips are used to cut dentin quickly and effectively, requiring less time than regular stainless steel or zirconium nitride inserts in similar preparations (Figure 2).
The diamond-coated tips have an abrasive effect on dentin and are useful to remove pulp stones and calcifications, to locate canals, to remove secondary dentin, and to clean isthmus areas.
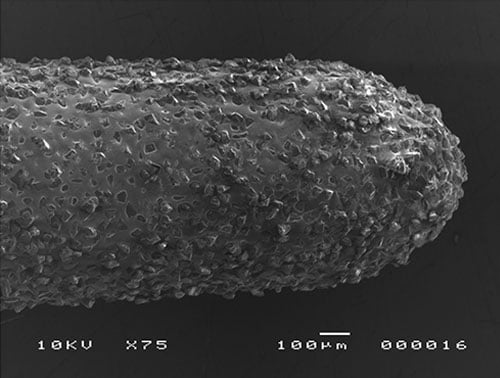
Figure 2: MEV image from a diamond-coated ultrasonic tip
Figure 3: MEV image from a non-diamond-coated ultrasonic tip
Standard tips
Standard tips are mostly used when it is necessary to preserve as much tooth structure as possible, as these cut less aggressively than diamond-coated tips. They are most useful to remove posts, separated instruments, gutta percha, cement, foreign bodies, debris, and smear layer through activation of irrigants in the canal system.
Main causes of failure in endodontic treatments
One of the main causes of failed endodontic treatments is the presence of bacterial biofilm in the root canal system, especially inside areas of complex anatomy and lateral canals.5 As a file — whether it is a hand file or NiTi rotary — is only able to reach the main canal and is unable to instrument any lateral anatomy, irrigation is key to endodontic success to clean bacteria and pupal tissue that the file cannot contact. A root canal left undiscovered or contaminated is likely to be the reason for the treatment failure.
The persistent endodontic infection may be due to the difficulty in reducing the microbial load below a certain threshold. These microorganisms (isolated or within a biofilm) may be located in the ramifications of the root canal, including isthmus areas.6 According to one micro-CT study, 85% of all molars have isthmus areas.7 Therefore, the presence of difficult access anatomic areas is not an exception, but a rule. The current micro-CT studies require an anatomic pairing before the research itself. It is remarkable that many times the isthmus area is larger than the root canal area. With that in mind, in order to reduce the microbial load below the necessary threshold, it is imperative to thoroughly clean these areas using irrigation combined with some type of agitation process (mechanic, sonic, ultrasonic, or laser agitation).
Besides the clinical diagnosis and the case planning, knowing the root canal morphology and its many potential variations is fundamental to achieving success in endodontic treatment.8 When dealing with C-shaped canals, rotary and reciprocating instruments can easily shape the canals, which can then be cleaned and disinfected with the appropriate irrigating solutions. But many research papers have shown that mechanical preparation does not contact the entire canal system.1-3,9-11 On top of that, during preparation the debris produced when cutting the dentin walls of the canal are pushed inside lateral canals and isthmus areas (Figure 3).
The problem is quite clear: The vast majority of root canals do not have a simple anatomy. There is a high occurrence of flattened and oval-shaped canals, especially in mandibular molars; and the available rotary/reciprocating systems cut dentin in a conical shape, leaving a considerable percentage of untouched areas.1-3
Presence of isthmus
With the exception of upper anterior teeth, the isthmus is a common anatomic structure in human teeth. The isthmus can be described as a small horizontal groove that unites two root canals and can run all the way to the root or be limited to the coronal or mid portion of the canal, or even be present only at the apical portion. The isthmus is an area difficult clean and decontaminate with mechanical preparation due to its perpendicular position to the file, and it can have a major impact in the success of a root canal treatment.5,8
In a clinical study,12 1,400 teeth from 618 patients were evaluated using cone beam computed tomography. The study demonstrated that 87.9% of mandibular molars have isthmus present (Figure 4).
A high prevalence of isthmus areas means the practitioner must pay special attention during the preparation of mandibular molars. The use of ultrasonics and optical magnification is a fundamental tool to locate, identify, and clean this intricate anatomy.
Besides the high percentage of isthmus areas in mandibular molars, another clinical difficulty is the presence of a third canal in the mesial root, the middle mesial canal. This canal is located between the mesiobuccal and mesiolingual canals. The entrance point for the middle mesial canal is very hard to find without the use of magnification; however, in studies using the operative microscope, the canal was found in 46% of the investigated molars.13
The instrumentation, either manual or mechanized, is restricted by the main canal diameter, having little or no effect on flattened areas and twists/curvature of the root canal. Specially designed ultrasonic instruments allied to ultrasonic cavitation can favor the treatment inside flattened regions — raising the percentage of touched wall areas by the activated irrigants and performing a thorough cleaning without promoting excessive loss of tooth structure.
Protocol to prepare the mesial root canals in mandibular molars (Figure 5)
- After preparing the mesiobuccal and mesiolingual canals, remove the dentin over the isthmus using a diamond-coated ultrasonic tip. This procedure is best accomplished with a thick cone, pear, or sphere-shaped tip.
- Once the isthmus is visible through magnification, initiate its cleaning using a thin tip such as the E18D diamond-coated ultrasonic tip (Helse Ultrasonic, Santa Rosa de Viterbo, Brazil) or the UCT-1 diamond-coated ultrasonic tip (Vista Dental, Racine, Wisconsin) or the BUC-1A (Kerr Dental, Orange, California).
- In addition to cleaning this area, we must look for the middle mesial canal, which is generally located closest to the mesiolingual than to the mesiobuccal canal.
- Once the entrance is found, prepare the canal with carbon steel hand files, since they have a proper stiffness for this situation. A No. 6 carbon steel file is ideal for initial exploration of the canal due to its greater stiffness compared to a stainless steel file of the same size.

Figure 4: Presence of debris in the buccal and lingual areas of a distal mandibular molar after the use of NiTi rotary files
Figure 5: Micro-CT showing high prevalence of flattened canals and isthmus areas 
Figure 6: A. Diagnostic X-Ray kindly provided by Dr. Tiago Braga; B. Image taken after access surgery showing the mesiobuccal, mesiolingual, and distal canals already prepared; C. Middle mesial canal entrance located using an pear-shaped diamond-coated ultrasonic tip; D. Middle mesial canal after bio-mechanical preparation; E. Final X-ray showing preparation for a post in the distal root; F. Inverted X-ray image.
Protocol to prepare the distal root in mandibular molars
After having prepared the distal root using rotary or reciprocating files, use an ultrasonic tip to clean the buccal and lingual walls, touching every area that hasn’t been cleaned by the files. This procedure is better accomplished with a thin diamond-coated tip (examples: E2D diamond-coated ultrasonic tip, Helse Ultrasonic, Santa Rosa de Viterbo, Brazil; CKT2 Diamond, diamond-coated ultrasonic tip, Excellence in Endodontics, San Diego, California; or CPR-2D ultrasonic tip Kerr Dental, Orange, California). The basic idea is using the ultrasonic tips to remove debris and dissociate biofilm in the areas left untouched by conventional instruments, allowing a greater penetration of the irrigating solutions (Figure 6).
Figure 7: A. Flattened distal canal of a mandibular molar; B. Same tooth after preparation using rotary instruments; C. Note the red line over the area left untouched by the rotary files; D. Same canal after using an E18D diamond-coated ultrasonic tip (Helse Ultrasonic, Santa Rosa de Viterbo, Brazil.) (Alternative tips: CPR-4D ultrasonic tip, Kerr Dental, Orange, California or UCT-1 ultrasonic tip, Vista Dental, Racine, Wisconsin)
Figure 8: E1 Irrisonic ultrasonic tip
Figure 9: IrriSafe ultrasonic tip
Apical region
According to van der Sluis, Versluis, and Wesselink,14 using ultrasonic activated irrigation to clean the root canal system, including the apical region, is as effective as a K-file when dealing with curved canals, provided that a properly designed ultrasonic tip is used, one that is flexible enough to go 2 mm short of the working length. That way, the irrigation solution can be pushed into the isthmus areas, thus removing dentin debris, tissue remains, and biofilm. The application of ultrasonic energy to agitate the irrigating solution has shown great effectiveness to clean isthmus and other difficult access areas.15,16 The E1 Irrisonic™ ultrasonic tip (Helse Ultrasonic, Santa Rosa de Viterbo, Brazil, Figure 8) and the IrriSafe™ ultrasonic tip (Satelec Acteon, Figure 9) were especially developed to activate the irrigation solution in the whole root canal system, including the apical region.
The agitation of the irrigation solution must be done with the ultrasonic hand piece placed by the buccal side of the tooth. That will help to enhance the cleaning process, since it will follow the isthmus anatomy.17
Recommended ultrasonic activation protocol:
- Fill the canals and the chamber with 17% EDTA solution and activate with the ultrasonic tip for 15 seconds.
- Rinse the canal with water, apply suction, and then refill the canals and the chamber again with sodium hypochlorite, and activate the solution with the ultrasonic tip for 15 seconds.
- Repeat steps 1 and 2 until the solution in the chamber remains clear (absence of cloudiness related to debris).
The ultrasonic agitation can also be applied to calcium hydroxide (CaOH2) paste in order to potentiate its penetration into the dentinal tubules and accessory canal anatomy, raising the alkalinity and resulting in better asepsis.18,19 The same ultrasonic tip is recommended to disperse the CaOH2 paste.
Calcium hydroxide paste activation protocol:
- Fill the root canal with the calcium hydroxide paste and agitate it with the ultrasonic tip for 30 seconds in the buccal-lingual direction.
- Fill the root canal with additional paste and agitate it for another 30 seconds, this time in the mesial-distal direction.
- If necessary, repeat steps 1 and 2.
The ultrasonic unit can also be used to agitate the obturation sealer, to do lateral condensation, and to cut the gutta percha cone.
The agitation of the sealer results in a greater penetration into dentinal tubules and accessory anatomy and promotes a higher adhesion of epoxy and MTA-based sealer.20,21
To perform lateral condensation using ultrasonics, it is necessary to choose an ultrasonic tip with the ability to thermoplasticize gutta percha. These tips also create room for accessory cones, enhancing the obturation homogeneity. Finally, a cylinder-shaped ultrasonic tip like the E10 ultrasonic tip (Helse Ultrasonic, Santa Rosa de Viterbo, Brazil) or the CT4 ultrasonic tip (Vista Dental, Racine, Wisconsin) is recommended for cutting the obturation cones at the canal entrance level.
Figure 10: Root canals where the obturation sealer was activated using an ultrasonic tip and the gutta-percha cones were thermoplasticized also with the aid of ultrasonics 
Figure 11: A. Pulp chamber after access — regular canal with gutta percha; B. Ultrasonic tip removing the gutta percha from the canal; C. Remaining gutta percha in the apical portion after using the ultrasonic approach
Protocol to activate the obturation sealer
- Fill the root canal with obturation sealer using a file or a Lentulo spiral, and agitate it with the ultrasonic tip for 30 seconds in the buccal-lingual direction.
- Fill the root canal with obturation sealer again, and agitate for another 30 seconds, this time in the mesial-distal direction, and insert the main cone coated in sealer to working length. The ultrasonic tip is activated and inserted into the canal alongside the cone that has been placed to condense the obturation material and create space for accessory cones.
- When space allows, accessory cones are coated with additional sealer and inserted, then condensed with the ultrasonic tip until the canals are fully obturated.
- An E10 or equivalent tip is utilized to cut off any cones protruding above the canal orifice.
Retreatment
Different retreatment techniques have been proposed for endodontic retreatment, including hand files with or without the use of chemical solvents22-24, rotary nickel-titanium systems, reciprocating systems, and adaptive motion systems. However, none of the existing techniques and file systems were able to completely remove the filling material from the canals.22-27 This has been shown to occur mainly in cases with anatomical complexities like oval-shaped and flattened canals27 or presence of an isthmus.28 In this way, several supplementary techniques have been suggested as additional approaches to improve root filling removal during endodontic retreatment, such as the use of ultrasonic tips26,29 and the XP-Endo Finisher (Martensite-Austenite ElectropolishFleX, FKG Dentaire).30 At first, a R1 Clearsonic ultrasonic tip (Helse Ultrasonic; no similar tips found from other manufacturers) can be used to remove gutta percha from the coronal and middle portions of the root canal. The heat produced by the ultrasonic energy is enough to remove the gutta percha during retreatments without the need for solvents. The cone-shaped tip end allows the practitioner to “fish” the already softened gutta-percha mass out of the canal. This method is cleaner and faster than using manual files. Another advantage is related to the canal anatomy, which is kept intact by the ultrasonic tip — it removes filling material without removing dentin (Figure 10).
After removing the gutta percha from the coronal and middle portions of the root canal, the remaining obturation inside the apical portion must be removed with an XP-Shaper .04/30 instrument (Martensite-Austenite ElectropolishFleX, FKG Dentaire). The XP-Shaper should be used at 1000 RPMs after having used #10 and #15 manual files, through wide forward and backward rotary movements, in order to remove the gutta percha that is still attached to the canal walls. The instrument tip must go all the way from the coronal to the apical portion. After using the XP-Shaper, perform an abundant irrigation followed by suction.
To finalize the gutta percha and cement removal, it is necessary to perform the ultrasonic activation of the irrigation solution in order to detach the remaining obturation material that is still holding to the root canal walls. The combination of ultrasonic tips and XP-Shaper instruments allow a much more complete removal of gutta percha and sealer from the main canals and also from the secondary anatomy.
Recommended ultrasonic activation protocol:
- Fill the chamber with EDTA and activate the solution with the ultrasonic tip for 15 seconds.
- Rinse the canal, apply suction, and then flood it again — this time with sodium hypochlorite — and activate the solution with the ultrasonic tip for 15 seconds.
- Repeat steps 1 and 2 until all of the existing gutta percha and sealer has been removed.
- Check for any gutta-percha remains.
- If there are gutta-percha remains, they can be removed using a thin tip like the E18D diamond coated ultrasonic tip (Helse Ultrasonic, Santa Rosa de Viterbo, Brazil) or the BUC-1A ultrasonic tip (SybronEndo, Orange, USA). These tips or their equivalents, because of the reduced diameter, are more likely to penetrate difficult access regions.
A complete cleaning of the root canal walls can be achieved through the use of ultrasonic tips combined with magnification and after the mechanical removal of gutta percha using NiTi rotary or reciprocating instruments.26,31 Hence, the most effective retreatment technique available today combines mechanical instrumentation, magnification, and ultrasonics.
Conclusion
An endodontic treatment that fails to remove biofilm in the root canal system is highly likely to become a failed endodontic treatment. Identifying and cleaning difficult access areas and accessory canals is very important to prevent failure and the need for future retreatment. Among all available options, the combination of mechanical files, ultrasonic tips, and magnification is an inexpensive and highly effective way to boost the success rate of one’s root canal treatments. And, when a retreatment is needed, the same combination can be used to remove obturation materials without having the negative side of using solvents.
References
- Paqué F, Balmer M, Attin T, Peters OA. Preparation of oval-shaped root canals in mandibular molars using nickel-titanium rotary instruments: a micro-computed tomography study. J Endod. 2010;36(4):703-707.
- Siqueira JF Jr, Alves FR, Almeida BM, de Oliveira JC, Rôças IN. Ability of chemomechanical preparation with either rotary instruments or self-adjusting file to disinfect oval-shaped root canals. J Endod. 2010;36(11):1860-1865.
- Peters OA, Boessler C, Paqué F. Root canal preparation with a novel nickel-titanium instrument evaluated with micro-computed tomography: canal surface preparation over time. J Endod. 2010;36(2):1068-1072.
- Villas-Bôas MH, Bernardineli N, Cavenago BC, et al. Micro-computed tomography study of the internal anatomy of mesial root canals of mandibular molars. J Endod. 2011;37(12):1682-1686.
- Ricucci D, Siqueira JF Jr, Bate AL, Pitt Ford TR. Histologic investigation on root canal treated teeth with apical periodontitis: a retrospective study from twenty-four patients. J Endod. 2009;35(4):493-502.
- Nair PN. On the causes of persistent apical periodontitis: a review. Int Endod J. 2006;39(4):249-281.
- Fan B, Pan Y, Gao Y, Fang F, Wu Q, Gutmann JL. Three-dimensional morphologic analysis of isthmuses in the mesial roots of mandibular molars. J Endod. 2010;36(11):1866-1869.
- Carr GB, Schwartz RS, Schaudinn C, Gorur A, Costerton JW. Ultrastructural examination of failed molar retreatment with secondary apical periodontitis: an examination of endodontic biofilms in an endodontic retreatment failure. J Endod. 2009;35(9):1303-1309.
- Peters OA, Paqué F. Root canal preparation of maxillary molars with the self-adjusting file: a micro-computed tomography study. J Endod. 2011;37(1):53-57.
- Zhao D, Shen Y, Peng B, Haapasalo M. Root canal preparation of mandibular molars with 3 nickel-titanium rotary instruments: a micro-computed tomographic study. J Endod. 2014;40(11):1860-1864.
- Duque JA, Vivan RR, Cavenago BC, et al. Influence of NiTi alloy on the root canal shaping capabilities of the ProTaper Universal and ProTaper Gold rotary instrument systems. J Appl Oral Sci. 2017;25(1):27-33.
- Estrela C, Rabelo LE, de Souza JB, et al. Frequency of root canal isthmi in human permanent teeth determined by cone-beam computed tomography. J Endod. 2015;41(9):1535-1539.
- Azim AA, Deutsch AS, Solomon CS. Prevalence of middle mesial canals in mandibular molars after guided troughing under high magnification: an in vivo investigation. J Endod. 2015;41(2):164-168.
- van der Sluis LW, Versluis M, Wu MK, Wesselink PR, et al. Passive ultrasonic irrigation of the root canal: a review of the literature. Int Endod J. 2007;40(6):415-426.
- Burleson A, Nusstein J, Reader A, Beck M. The in vivo evaluation of hand/rotatory/ultrasound instrumentation in necrotic, human mandibular molars. J Endod. 2007; 33(7):782-787.
- Gutarts R, Nusstein J, Reader A, Beck M. In vivo debridement efficacy of ultrasonic irrigation following hand-rotary instrumentation in human mandibular molars. J Endod. 2005;31(3):166-170.
- Jiang LM, Verhaagen B, Versluis M, van der Sluis LW. Influence of the oscillation direction of an ultrasonic file on the cleaning efficacy of passive ultrasonic irrigation. J Endod. 2010;36(8):1372-1376.
- Duarte MA, Balan NV, Zeferino MA, et al. Effect of ultrasonic activation on pH and calcium released by calcium hydroxide pastes in simulated external root resorption. J Endod. 2012;38(6):834-837.
- Arias MP, Maliza AG, Midena RZ, Graeff MS Duarte MA, Aandrade FB. Effect of ultrasonic streaming on intra-dentinal disinfection and penetration of calcium hydroxide paste in endodontic treatment. J Appl Oral Sci. 2016;24(6):575-581.
- Guimarães BM, Amoroso-Silva PA, Alcalde MP, Marciano MA, de Andrade FB, Duarte MA. Influence of ultrasonic activation of 4 root canal sealers on the filling quality. J Endod. 2014;40(7):964-968.
- Wiesse PEB, Silva-Sousa YT, Pereira RD, et al. Effect of ultrasonic and sonic activation of root canal sealers on the push-out bond strength and interfacial adaptation to root canal dentine. Int Endod J. 2017. https://onlinelibrary.wiley.com/wol1/doi/10.1111/iej.12794/full
- Horvath SD, Altenburger MJ, Naumann M, Wolkewitz M, Schirrmeister JF. Cleanliness of dentinal tubules following gutta-percha removal with and without solvents: a scanning electron microscopic study. Int Endod J. 2009;42(11):1032-1038.
- Roggendorf MJ, Legner M, Ebert J, Fillery E, Frankenberger R, Friedman S. Micro-CT evaluation of residual material in canals filled with Activ GP or Gutta Flow following removal with NiTi instruments. Int Endod J. 2010;43(3):200-209.
- Barreto MS, da Rosa RA, Santini MF, et al. Efficacy of ultrasonic activation of NaOCl and orange oil in removing filling. J Appl Oral Sci. 2016;24(1):37-44.
- Barletta FB, Rahde Nde M, Limongi O, Moura AA, Zanesco C, Mazocatto G. In vitro comparative analysis of 2 mechanical techniques for removing Gutta-Percha during retreatment. J Can Dent Assoc. 2007;73(1):65.
- Bernardes RA, Duarte MA, Vivan RR, Alcalde MP, Vasconcelos BC, Bramante CM. Comparison of three retreatment techniques with ultrasonic activation in flattened canals using micro-computed tomography and scanning electron microscopy. Int Endod J. 2016;49:890-897.
- Crozeta BM, Silva-Sousa YT, Leoni GB, et al. Micro-computed tomography study of filling material removal from oval-shaped canals by using rotary, reciprocating, and adaptive motion systems. J Endod. 2016;42(5):793-797.
- Cavenago BC, Ordinola-Zapata R, Duarte MA, et al. Efficacy of xylene and passive ultrasonic irrigation on remaining root filling material during retreatment of anatomically complex teeth. Int Endod J. 2014;47(11):1078-1083.
- Fruchi LC, Ordinola-Zapata R, Cavenago BC, Hungaro Duarte MA, Bueno CE, De Martin AS. Efficacy of reciprocating instruments for removing filling material in curved canals obturated with a single-cone technique: a micro-computed tomographic analysis. J Endod. 2014;40(7):1000-1004.
- Alves FR, Marceliano-Alves MF, Sousa JC, Silveira SB, Provenzano JC, Siqueira JF Jr Removal of root canal fillings in curved canals using either reciprocating single- or rotary multi-instrument systems and a supplementary step with the XP-Endo Finisher. J Endod. 2016;(42)(7)1114-1119.
- Ma J, Al-Ashaw AJ, Shen Y, et al. Efficacy of ProTaper Universal Rotary Retreatment system for gutta-percha removal from oval root canals: a micro-computed tomography study. J Endod. 2012;38(11):1516-1520.
Stay Relevant With Endodontic Practice US
Join our email list for CE courses and webinars, articles and more..


